Chemical Bonds
Chemical Bonds
This lesson introduces bonding and explains the three ways in which atoms can become stable. The rest of the lesson examines different types of bonds in more detail.
The following video will provide a review on Chemical Bonds.
Introduction to Bonding
Chemical elements found in the periodic table have different levels of reactivity. The number of valence electrons in an atom is the most important factor in determining how an element will react. Valence electrons, which are found in an atom’s outermost energy level, are involved in forming chemical bonds. The periodic table below shows the Bohr models of select elements. The valence electrons appear in red.
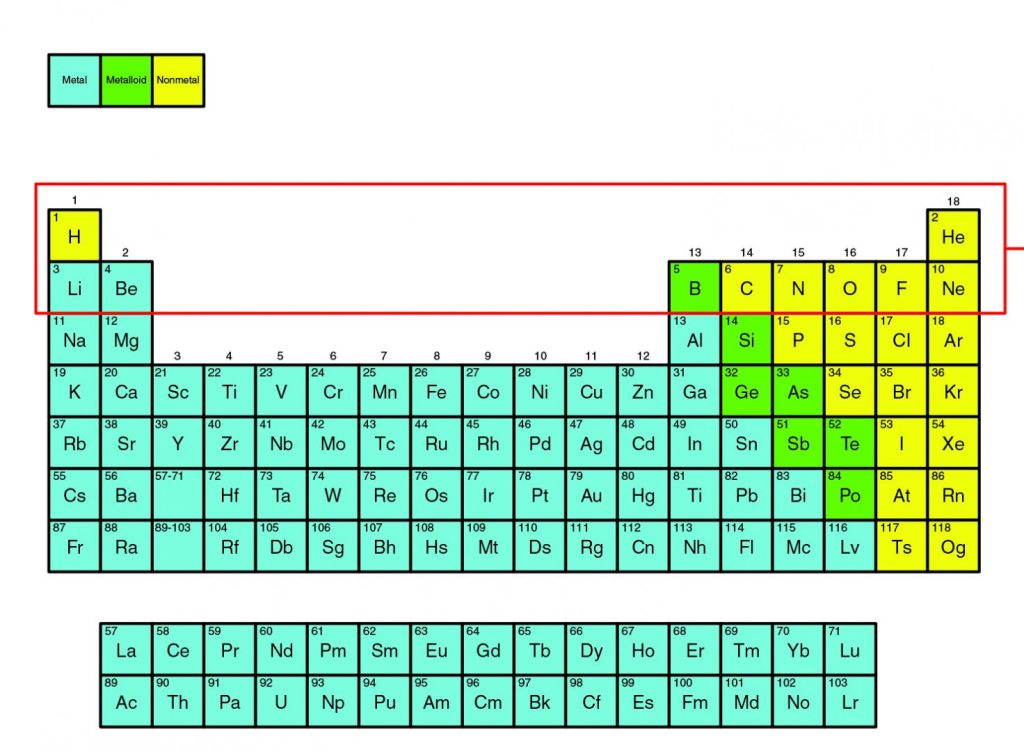
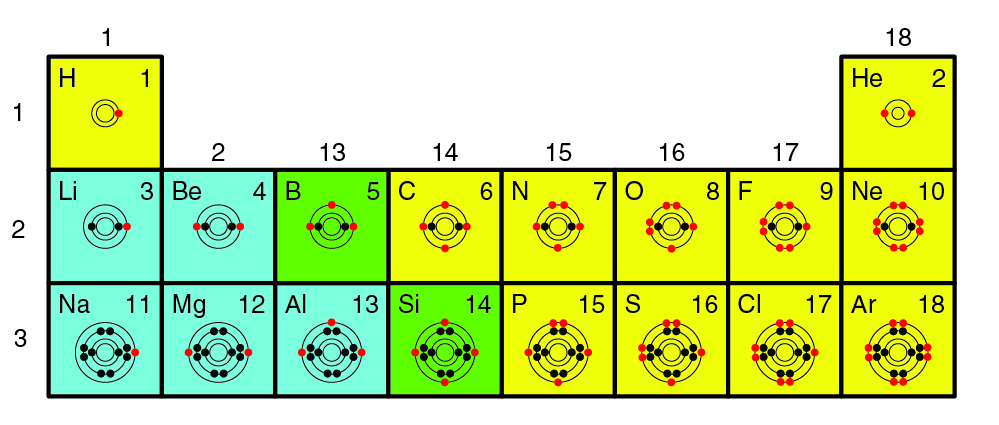
The octet rule states that atoms will lose, gain, or share electrons to obtain a stable electron configuration of eight valence electrons. In other words, if an atom needs to become stable, it will react with another atom, which can result in the formation of a chemical compound. Note that the elements in group 18, the noble gases, have eight valence electrons. Helium is an exception and is stable with two valence electrons. Because they have a stable electron configuration, the noble gases do not need to react with other elements to become stable. As a result, they are found in nature as single elements rather than in compounds.
Key Point

The goal of forming chemical bonds is to become stable by having eight electrons in the outer shell. This is easy to remember because it is described in the octet rule. The prefix octa- means “eight,” and it can be seen in other words, such as octopus and octagon.
Elements in other groups will react to become stable in predictable ways, depending on how many valence electrons they have. In the periodic table above, elements are classified as metals, nonmetals, or metalloids. Compared to other elements, metals have fewer valence electrons and tend to lose them to become stable. Notice that removing the red valence electrons from the outermost energy level exposes another energy level. This becomes the valence shell, and the atom is stable because it has eight valence electrons.

Nonmetals and metalloids have a relatively high number of valence electrons. Except for the noble gasses, these elements tend to gain or share electrons to become stable.
Ionic compounds are formed when electrons are transferred from a metal (which loses one or more electrons) to a nonmetal (which gains one or more electrons).
Covalent compounds are formed when two nonmetals or metalloids share electrons.

Test Tip

A quick way to determine if atoms are held together by ionic or covalent bonds is to examine the types of elements involved. If a metal and a nonmetal bond, an ionic bond forms. If two nonmetals or metalloids bond, a covalent bond forms.
Ion Formation
If an atom has an equal number of positively charged protons and negatively charged electrons, it is neutral and has no net charge. When electrons are transferred, atoms end up with either more protons than electrons or more electrons than protons. The atoms are considered ions because they have a net positive or negative charge.
When a metal such as sodium reacts to become stable, it loses its valence electrons. At first, it is a neutral atom with 11 protons and 11 electrons. When it loses an electron, the number of protons does not change, and the atom has 11 protons and 10 electrons. Because there is one more positively charged proton, a cation forms. A cation is an ion with a net positive charge.
Keep in Mind

Protons are positively charged subatomic particles and can be represented by the symbol p+. Electrons are negatively charged particles and can be represented by the symbol e-.

When a nonmetal such as chlorine reacts to become stable, it gains a valence electron. At first, it is a neutral atom with 17 protons and 17 electrons. When it gains an electron, the number of protons does not change, and the atom has 17 protons and 18 electrons. Because there is one more negatively charged electron, an anion forms. An anion is an ion with a net negative charge.
Be Careful!
When an atom gains electrons, it has a net negative charge because it gains negatively charged particles. When an atom loses electrons, it has a net positive charge. After the loss, there are more protons than electrons, which means there are more positively charged particles.
The way in which an element reacts can be predicted based on that element’s position in the periodic table. The table below summarizes the reactivity of elements according to their group number. Elements in each group have a specific number of valence electrons, which dictates what the atoms need to do to obtain a valence shell with eight electrons. Some will lose electrons, and others will gain electrons. This, along with the number of electrons that must be transferred, determines the charge of the stable ion that forms.
| Group | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|
| Valence e- | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Lose/Gain e- | Lose 1 | Lose 2 | Lose 3 | Lose/Gain 4 | Lose/Gain 3 | Lose/Gain 2 | Lose/Gain 1 | N/A |
| Charge | +1 | +2 | +3 | +4/- | -3 | -2 | -1 | N/A |
Ionic Bonding
An ionic compound is composed of a cation and an anion. An ionic bond is formed from the cation’s attraction to the oppositely charged anion. The figure below shows how transferring an electron from sodium to chlorine results in the formation of an ionic bond.
Notice that the charges on sodium (Na+) and chlorine (Cl–) ions have the same magnitude (they both have a value of 1). Therefore, the charge of one sodium ion balances the charge of one chlorine ion. When an ionic compound is formed from ions that have equal but opposite charges, the elements will be present in a 1:1 ratio. Examples are shown in the table below.

| Compound Name | Cation | Anion | Compound Formula |
| Potassium fluoride | K+ | F– | KF |
| Magnesium oxide | Mg2+ | O2- | MgO |
| Aluminum nitride | Al3+ | N3- | ALN |
In some cases, atoms need to lose or gain two, three, or, in rare cases, four electrons to become stable. For example, magnesium must give up two electrons to become stable. Because chlorine only needs one electron, magnesium can give an electron to two different chlorine atoms. Then, one magnesium cation with a +2 charge (Mg2+) bonds with two chloride anions (Cl–) to form magnesium chloride, (MgCl2). The subscript 2 indicates that there are two chloride ions in this compound.
| Compound Name | Cation | Anion | Compound Formula |
|---|---|---|---|
| Calcium bromide | Ca2+ | Br– | CaBr2 |
| Aluminum fluoride | Al3+ | F– | AlF3 |
| Rubidium oxide | Rb+ | O2- | Rb2O |
| Sodium phosphide | Na+ | P3- | Na3P |
| Aluminum oxide | Al3+ | O2- | Al2O3 |
| Calcium phosphide | Ca2+ | P3- | Ca3P2 |
Similarly, when oxygen and lithium react, the oxygen atom receives an electron from each of two lithium atoms. This transfer results in two lithium cations (Li+) and an oxygen anion (O2-). They attract each other to form the compound lithium oxide with a formula of Li2O. Other examples are shown in the table below. Notice that in all ionic compounds, the total positive charge balances out the total negative charge, resulting in a neutral compound.
Key Point

Regardless of how many electrons are transferred, ionic compounds have net charges of zero. They are all neutral because the positive cations attract as many anions as they need to balance their charges, and vice versa.
Covalent Bonding

When a nonmetal atom reacts with a nonmetal or metalloid, the atoms share electrons to obtain eight valence electrons each. An example can be seen in the model below. Both the Bohr models and the electron dot structures of the fluorine atoms show their seven valence electrons. After each atom shares an electron with the other, shown by the arrows, a covalent bond forms. In the newly formed fluorine molecule, both fluorine atoms have the stable electron configuration of eight valence electrons. The shared electrons can be represented by two dots or by a line in between the fluorine atoms.
Covalent compounds can be modeled in Lewis structures. Lewis structures for methane, ammonia, and water are shown below. In a Lewis structure, covalent bonds, also called shared electrons, are represented by lines between two atoms. Valence electrons that are not involved in bonding, also called lone-pair electrons, are represented by dots.
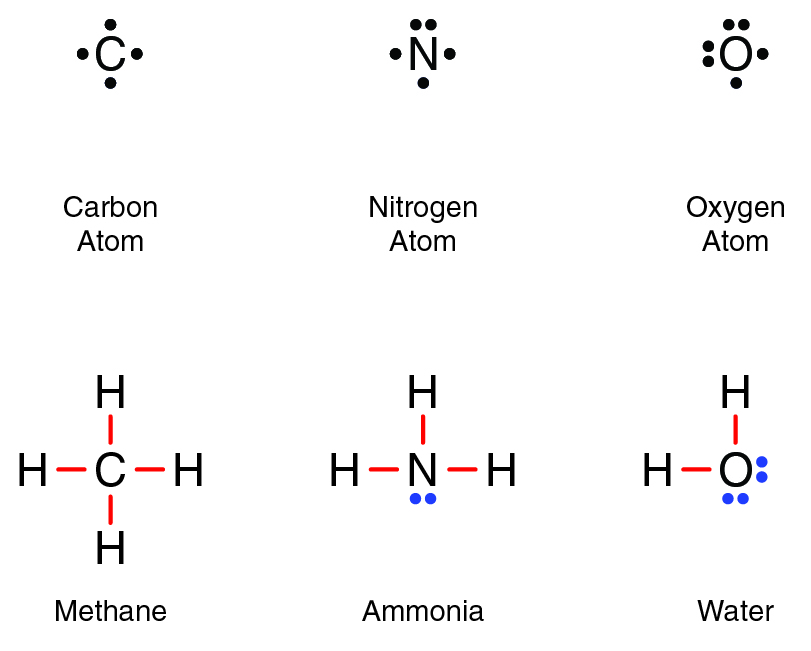
The number of bonds that an atom forms depends on the number of valence electrons that the atom has as a single atom. In a molecule of methane (CH4), one carbon atom bonds to four hydrogen atoms. A single neutral carbon atom has four valence electrons and can share each one with a different hydrogen atom. In the end, it has four covalent bonds. Because each covalent bond involves two electrons, carbon has a total of eight valence electrons and is stable.

Keep In Mind
Each line (bond) in a Lewis structure represents two electrons, one from each atom involved in the bond.
Similarly, in a molecule of ammonia (NH3), one nitrogen atom bonds to three hydrogen atoms. Nitrogen shares six electrons total and has two remaining lone-pair electrons that are not involved in bonding for a total of eight. In a water molecule, an oxygen atom bonds to two hydrogen atoms. Oxygen has four shared electrons and four lone-pair electrons for a total of eight.
Types of Covalent Bonds
In methane, ammonia, and water, atoms are joined by single covalent bonds in which the atoms share two electrons. However, two atoms may need to share more than one pair of electrons to be stable. For example, two oxygen atoms form a double bond, in which two pairs of electrons (four electrons total) are shared. Similarly, two nitrogen atoms form a molecule with a triple bond, in which three pairs of electrons (six electrons total) are shared.

As more pairs of electrons are shared, the length of the bond decreases, and the bond strength increases. Single bonds are the longest and weakest bonds. They require the least energy to break because there is not as much energy stored in them.
Connection

A pair of shared electrons between two atoms can be compared to a rubber band stretching between two objects.
Having two or three rubber bands, rather than one, increases the strength of the “bond” that holds them together, making it harder to separate the objects.
Regardless of how many electrons are shared, the strength of a covalent bond comes from the positively charged nuclei of both atoms attracting the negatively charged electrons that are being shared. However, not all atoms attract shared electrons equally. This property is known as electronegativity, the tendency of an atom to attract shared electrons in a covalent bond. It is a measure of how hard an atom is pulling on shared electrons. Electronegativity increases going from left to right in the periodic table. Nonmetal atoms pull harder on electrons and do not tend to give them up. Therefore, the halogens in group 17 have the highest electronegativity of all elements.
If the two atoms share electrons equally, the bond is classified as nonpolar covalent. This occurs if the two atoms have similar electronegativities, which means that neither atom pulls significantly harder on the shared electrons than the other. If the two atoms share electrons unequally, the bond is polar covalent. This occurs if the electronegativity of one atom is significantly higher than the other, causing it to pull significantly harder on the shared electrons.

Connection
The sharing of electrons is like a game of tug-of-war in which two opposing teams are pulling on a rope in opposite directions. In a nonpolar bond, the opposing teams are pulling with the same force, and the rope is not moving toward one team or the other. In a polar bond, one team is winning by pulling the rope closer to its side.
Let’s Review
- The periodic table of elements organizes elements in order of increasing atomic mass and by other characteristics. Elements have the number of energy levels equivalent to the row they are in. Elements in the same column of the periodic table have the same number of valence electrons in the last energy level.
- As stated in the octet rule, any atom that does not have a stable electron configuration of eight valence electrons will lose, gain, or share electrons to become stable.
- Exceptions to the octet rule include hydrogen and helium, which are stable when they have two valence electrons. This is because the first energy level of the electron cloud can fit two electrons.
- Ionic bonds are formed when electrons are transferred from a metal atom to a nonmetal atom. When an atom gains electrons, it becomes an anion, because it has a net negative charge since it gains negatively charged particles. When an atom loses electrons, it’s called a cation because it has a net positive charge since the newly formed ion will have more protons than electrons.
- Covalent bonds are formed when two atoms share electrons. When two atoms need to share more than one pair of electrons, multiple bonds form. If two pairs are shared, a double bond forms. If three pairs are shared, a triple bond forms.
- The difference in the electronegativities of the two atoms determines if electrons are shared equally, forming a nonpolar covalent bond, or shared unequally, forming a polar covalent bond.
- Lewis structures are diagrams of atoms to represent bonding. Valence electrons and lone-pair electrons, or electrons not involved in bonding, are represented with dots while bonding patterns are represented with lines.
Subscribe to the online course to gain access to the full lesson content.
If your not ready for a subscription yet, be sure to check out our free practice tests and sample lesson at this link
