Chemical Solutions
Chemical Solutions
This lesson discusses the properties of different types of mixtures, focusing on solutions. Then, it examines aspects of chemical reactions, including the components of the reactions and the types of changes that occur.
The following videos will provide a review on the topics covered within Chemical Solutions.
Chemical Reactions Part I: Chemical Formulas
Chemical Reactions Part II: Types of Reactions
Chemical Solutions & Solubility Curves
Solutions
When elements and compounds are physically (not chemically) combined, they form a mixture. When the substances mix evenly and it is impossible to see the individual components, the mixture is described as homogeneous. When the substances mix unevenly and it is possible to see the individual components, the mixture is described as heterogeneous.
Solubility is the ability of a substance to dissolve in another substance. For example, salt and sugar are both substances that can dissolve in water. They are soluble. In contrast, sand does not dissolve in water. It is insoluble. Individual particles of sand can be seen in water, but individual particles of salt are completely mixed in.
When one substance dissolves in the other, a type of homogeneous mixture called a solution will form. The substance that is being dissolved is the solute. The substance in which the solute is dissolved is the solvent, which makes up a greater percentage of the mixture than the solute. When salt dissolves in water, salt is the solute, and water is the solvent. Saltwater is an example of an aqueous solution, which forms when any substance dissolves in water.
- Molarity (number of moles of a substance in one liter of solution)
- Molality (number of moles of a substance per kilogram of solvent)
- Percent composition by mass (mass of a solute per unit mass of the solution)
- Mole fraction (moles of a solute divided by the total number of moles in the solution)
Solubility can also refer to the amount of a substance that can dissolve. Even for soluble substances, there is a limit to how much of it can dissolve. The lines in the graph below show these limits for different substances at different temperatures in 100 grams of water. The area below a line represents masses of solute that dissolve in 100 grams of water. This type of solution is unsaturated because more solute can be dissolved. At the line, the solution is saturated because the limit has been reached. Any solute added above that mass will remain undissolved.

Connection
The term saturated is also used in everyday life to describe things, such as sponges or clothing, that have soaked up all the water that they can absorb.
Chemical Reactions
A chemical reaction involves elements and compounds that combine, break apart, rearrange, or change form in some way. Reactants are the substances that are present at the beginning of the reaction and undergo a change. Products are the substances that are formed from the reactants. Chemical reactions can be described by chemical equations, an example of which is shown below.
In this reaction, methane (CH4) is burned in the presence of oxygen (O2) to form carbon dioxide (CO2) and water (H2O). In the chemical equation, the formulas of the reactants (CH4 and O2) and products (CO2 and H2O) are used. If there is more than one reactant or more than one product, their formulas are separated by a plus sign (+). The reactants and products are separated by an arrow.
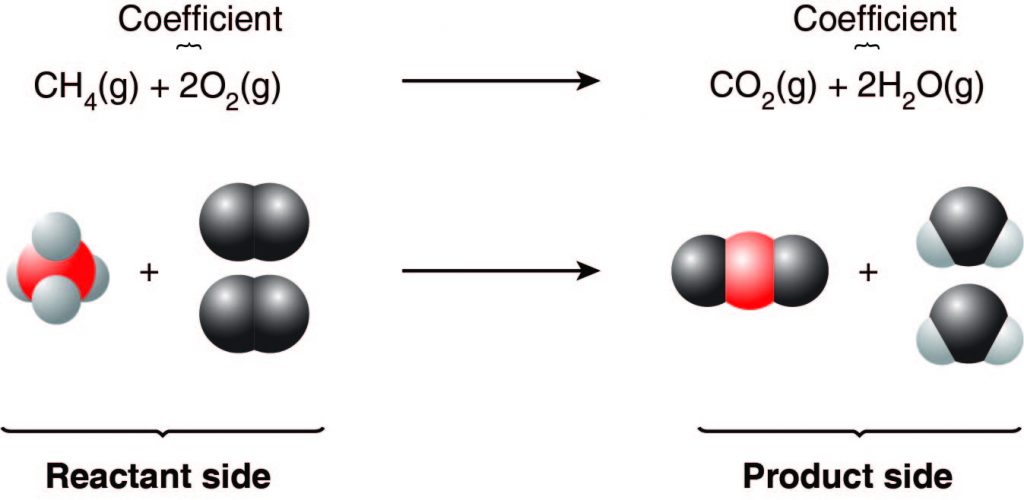
The state of matter may also be shown in the chemical equation in parentheses after the substance formula. Substances can be solid, liquid, or gas, indicated by (s), (l), or (g), respectively. If a substance is dissolved in water, forming an aqueous solution, that state is indicated by (aq) in a chemical equation.
Finally, coefficients may appear in chemical equations. These coefficients indicate how many particles (atoms or molecules) of each substance react or form. When there is no coefficient present, only one particle is involved. In the example above, one molecule of methane (CH4) reacts with two molecules of oxygen (O2). One molecule of carbon dioxide (CO2) is produced, along with two molecules of water (H2O).
Keep In Mind

Reactants will always be on the left side of the arrow, and products will always be on the right side.
Types of Reactions
Chemical reactions can be classified according to the reactants and products involved. This lesson will cover five types of reactions: synthesis, decomposition, single-replacement, double-replacement, and combustion. The first four types are outlined in the table below.
| Type of Reaction | Model | Example |
|---|---|---|
| Synthesis | A + B → AB | 2H2(g) + O2(g) → 2H2O(g) |
| Decomposition | AB → A + B | 2H2O2(aq) → 2H2O(l) + O2(g) |
| Single-Replacement | AB + C → AC + B | 2HCl(aq) + Zn(s) → ZnCl2(aq) + H2(g) |
| Double-Replacement | AB + CD → AD + CB | AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) |
Synthesis reactions involve two or more reactants (A and B) combining to form one product (AB). In the example provided, hydrogen (H2) and oxygen (O2) begin as separate elements. At the end of the reaction, the hydrogen and oxygen atoms are bonded in a molecule of water (H2O).
Decomposition reactions have only one reactant (AB) that breaks apart into two or more products (A and B). In the example above, hydrogen peroxide (H2O2) breaks apart into two smaller molecules: water (H2O) and oxygen (O2).
Single-replacement reactions involve two reactants, one compound (AB) and one element (C). In this type of reaction, one element replaces another to form a new compound (AC), leaving one element by itself (B). In the example, zinc replaces hydrogen in hydrochloric acid (HCl). As a result, zinc forms a compound with chlorine, zinc chloride (ZnCl2), and hydrogen (H2) is left by itself.
Double-replacement reactions involve two reactants, both of which are compounds made of two components (AB and CD). In the example, silver nitrate, composed of silver (Ag1+) and nitrate (NO31-) ions, reacts with sodium chloride, composed of sodium (Na1+) and chloride (Cl1-) ions. The nitrate and chloride ions switch places to produce two compounds that are different from those in the reactants.
Combustion reactions occur when fuels burn, and they involve specific reactants and products, as seen in the examples below. Some form of fuel that contains carbon and hydrogen is required. Examples of such fuels are methane, propane in a gas grill, butane in a lighter, and octane in gasoline. Notice that these fuels all react with oxygen, which is necessary for anything to burn. In all combustion reactions, carbon dioxide, water, and energy are produced. When something burns, energy is released, which can be felt as heat and seen as light.
| Fuel | Reaction |
|---|---|
| Methane (CH4) | CH4 + 2O2 → CO2 + 2H2O + energy |
| Propane (C3H8) | C3H8 + 5O2 → 3CO2 + 4H2O + energy |
| Butane (C4H10) | 2C4H10 + 13O2 → 8CO2 + 10H2O + energy |
| Octane (C8H18) | 2C8H18 + 25O2 → 16CO2 + 18H2O + energy |
Did You Know?

The fuels used in combustion reactions belong to a class of compounds called hydrocarbons because they are composed of hydrogen and carbon atoms. Hydrocarbons can be found in crude oil and include fossil fuels such as coal and natural gas. They are referred to as fossil fuels because they formed from the decomposition of organisms that died millions of years ago.
Energy Diagrams
Energy diagrams can be used to show how the energy of the species in a reaction changes over time. The reactants have a certain amount of energy stored in their bonds, and the products usually have a different amount of energy. If energy is released, the products have less energy than the reactants, and the reaction is exothermic. If energy is absorbed, the products have more energy than the reactants, and the reaction is endothermic. The shapes of the energy diagrams are shown below.

Connection

The difference between endothermic and exothermic reactions can be remembered by thinking about the meanings of the prefixes of these terms.
In an exothermic reaction, energy is released or “exits” the system.
In an endothermic reaction, energy “goes in.”
In every reaction, an activated complex must form between reactants. This complex can also be referred to as a transition state because it is required to convert, or provide a transition between, the reactants and products. In energy diagrams like the ones above, the activated complex has more energy than both the reactants and the products. The activation energy is the amount of energy required to transform the reactants into the activated complex, which then breaks apart to form the products.
The components of an energy diagram are as follows:
- Energy of reactants – energy of substances at the beginning of the reaction
- Energy of products – energy of substances at the end of the reaction
- Energy of the activated complex – energy of the substance represented by the maximum in the energy diagram
- Activation energy – difference in energy between the reactants and the activated complex
- Amount of energy released/absorbed – difference in energy between the reactants and products
Let’s Review
- A mixture is when elements and compounds are physically, but not chemically, combined.
- A homogeneous mixture is when substances mix evenly and it is impossible to see individual components. A heterogeneous mixture is when the substances mix unevenly and it is possible to see individual components.
- A solution is a type of homogeneous mixture that is formed when a solute dissolves in a solvent.
- The concentration of a solution is the amount of a substance in a given amount of solution. An unsaturated solution has the ability to dissolve more solute and a saturated solution has already reached the limit of solute it can dissolve.
- The law of conservation of mass states that matter cannot be created or destroyed, but it can change forms.
- Chemical reactions occur when reactants combine, break apart, or rearrange to form products.
- Chemical equations represent chemical reactions using formulas and symbols.
- Chemical equations must be balanced to obey the law of conservation of mass to ensure that all atoms at the beginning of the reaction in the reactants are accounted for at the end in the products.
- Chemical reactions can be classified as synthesis, decomposition, single-replacement, double-replacement, or combustion based on the reactants and products.
- Energy diagrams show how the energy of the species involved in the reaction changes as the reaction progresses.
Subscribe to the online course to gain access to the full lesson content.
If your not ready for a subscription yet, be sure to check out our free practice tests and sample lesson at this link
