Scientific Notation
Scientific Notation
This lesson begins by explaining how to convert measurements with very large or very small values into more manageable numbers using scientific notation. It then explores the structure of the atom and describes how to determine the number of protons, neutrons, and electrons in an atom of a specific element. Finally, it describes the relationship between isotopes of the same element and the effects that these isotopes have on the average atomic mass of an element.
Scientists often work with very large and very small numbers. For example, the radius of Earth’s orbit around the sun is very large: 15,000,000,000,000 centimeters. On the other extreme, the radius of a hydrogen atom is very small: 0.00000000529 centimeters. To make these numbers more manageable, scientists write them using scientific notation. Scientific notation is a way to represent numbers and contains three components, which are shown in the diagram below.
Understanding how these components relate to one another makes it possible to convert between standard notation and scientific notation. The coefficient is a number that has a value of at least 1 but less than 10 and includes all significant digits in the given value. Another way to think about this is that there should always be one non-zero digit before the decimal point.
In scientific notation, the base is always 10.

The exponent indicates the number of places the decimal point needs to move. Notice that when the exponent is positive, the decimal place moves to the right; this is how larger numbers are represented. When the exponent is negative, the decimal place moves to the left; this is how smaller numbers are represented. When the decimal must move beyond the digits that are in the measurement, the “empty” spaces are filled in with zeros.

Key Point
When converting from scientific notation to standard notation, a negative exponent requires the decimal point to move to the left, and a positive exponent requires the decimal point to move to the right.
The Atom
All matter is made of atoms. Every atom contains a dense core in the center called a nucleus. The nucleus is composed of subatomic particles called protons and neutrons. Surrounding this core is an area known as the electron cloud, in which smaller subatomic particles known as electrons are moving.
The Bohr model below shows these components of the atom. In the model, each subatomic particle is marked with a charge. Protons have a positive (+) charge, electrons have a negative (−) charge, and neutrons do not carry any charge; they are neutral. Therefore, the overall charge of an atom depends on the numbers of protons and electrons and is not influenced by the number of neutrons. An atom is neutral if the number of protons is equal to the number of electrons. If there are more protons than electrons, the atom will have an overall positive charge; if there are more electrons than protons, the atom will have an overall negative charge.

Compare the Bohr Model to a Real Atom
Note that this model is not to scale. The nucleus should be much smaller because it is about 10,000 times smaller than the electron cloud in a real atom. Also, the space between the electrons a real atom is much greater than in the model. In a real atom, the electron cloud is mostly empty space.
To further compare these three subatomic particles, their locations, charges, and masses are shown in the table below. The unit used for mass is the atomic mass unit (amu). The masses of a proton or neutron are considerably larger than the mass of an electron. This difference in mass has important implications. First, because the nucleus is so small relative to the overall size of the atom and contains the more massive protons and neutrons, it is extremely dense. Second, because the electrons are almost 2,000 times less massive than the other subatomic particles, they do not significantly influence the atom’s mass.
| Sub atomic Particle |
Symbol | Location | Charge | Mass (amu) |
|---|---|---|---|---|
| Proton | \(p+\) | Nucleus | +1 | 1.0 |
| Neutron | \(n^0\) | Nucleus | 0 | 1.0 |
| Electron | \(e-\) | Electron Cloud | -1 | 0.00054 |
One final note about the Bohr model of the atom is that the electrons lie on rings. These rings represent energy levels, sometimes referred to as electron “shells.” Electrons that occupy energy levels that are closest to the nucleus have the least energy. Electrons found farther from the nucleus have more energy. A limited number of electrons can occupy each energy level. The first energy level can fit up to 2 electrons. The second energy level can fit up to 8 electrons. The third energy level can fit up to 18 electrons. An atom in its normal state will have electrons lying in the lowest possible energy levels.
While the Bohr model provides a good way to visualize the atom, its representation of the electron cloud is not completely accurate. Electrons move around the nucleus in different energy levels, but this movement is not restricted to specific circular orbits as the Bohr model indicates. The quantum mechanical model (also known as the electron cloud model) describes the probable locations of electrons because their exact pathways, locations, and speeds cannot be determined simultaneously.
The Periodic Table of Elements
The atom is not only the basic building block of matter, but also the smallest unit of an element that can be defined as that element. All known elements are listed in the periodic table.

In the periodic table, elements are arranged in rows, also known as periods, and columns, also known as groups. Both the periods and the groups can be referred to by number. For example, argon is in period 3 and group 18.
Elements with similar properties are put into families that are outlined in different colors in the periodic table above. Note that these families generally correspond to the groups in the periodic table. For example, the elements in group 18 are in a family called the noble gases, while the elements in group 2 are all alkaline earth metals.
Periodic tables differ in the information they provide, and an example of a block is shown above. This block shows the name of the element and its chemical symbol, which is an abbreviation for the name. The chemical symbol is one, two, or three letters with the first letter capitalized and all subsequent letters letter lowercase. The symbol for the element argon is Ar.
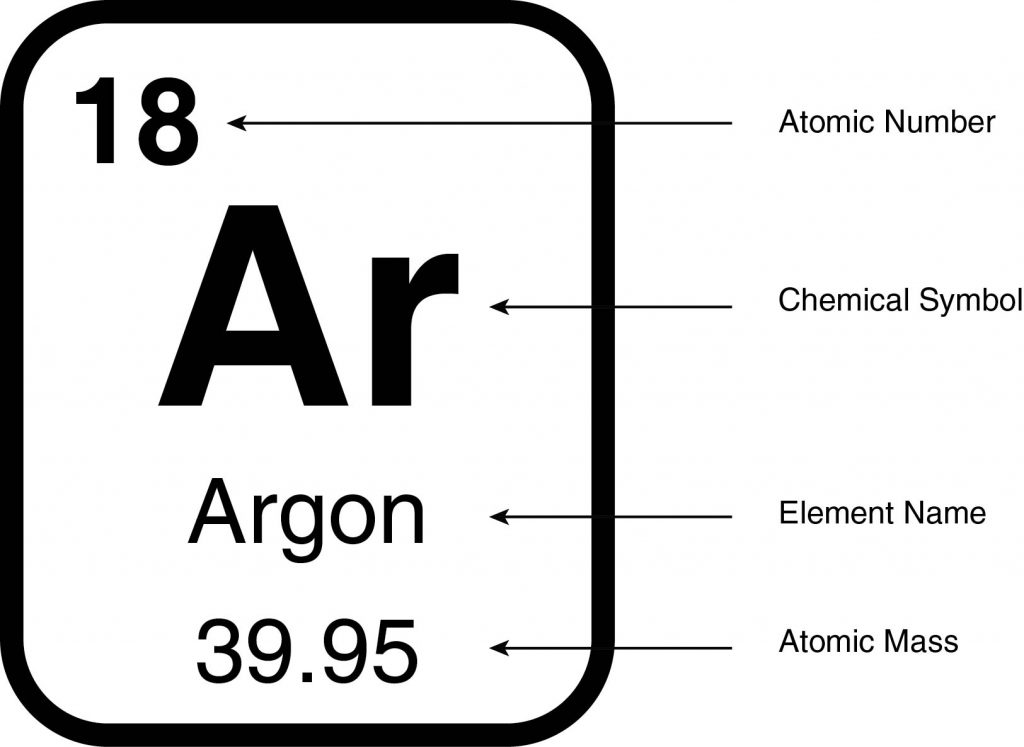

Did You Know?
While many elements have chemical symbols that resemble their names, like argon (Ar), some elements have chemical symbols that are different from their names. This is because the symbols are derived from either the Latin or the Greek names for the elements rather than the English names. The symbol for sodium is Na because the Latin name for the element is natrium.
Each element is assigned an atomic number. The atomic number is equal to the number of protons in a single atom of that element and is how an element is identified. Argon, for example, has an atomic number of 18. Therefore, every atom of argon has 18 protons, regardless of how many neutrons or electrons it has.
Average Atomic Mass and Mass Number
Some periodic tables also provide the average atomic mass of an element in atomic mass units (amu). Because not all atoms of argon have the same mass, the periodic table shows the average mass of all argon atoms. These forms of argon are differentiated based on their mass numbers, which are determined by adding the number of protons and neutrons. Argon has three stable forms, called isotopes, which are shown in the table below.
| Name | Abundance | Mass (amu) | Mass # |
# of Protons | # of Neutrons |
|---|---|---|---|---|---|
| Argon-36 | 0.337% | 35.97 | 36 | 18 | 18 |
| Argon-38 | 0.063% | 37.96 | 38 | 18 | 20 |
| Argon-40 | 99.6% | 39.96 | 40 | 18 | 22 |
The mass number can be used to determine the number of neutrons, as shown by the equation below. Argon-40 is the most abundant and has a mass number of 40. Its 18 protons contribute 18 to the mass number of the atom. The remaining mass is from the neutrons.
mass number = number of protons + number of neutrons
40 = 18 + number of neutrons
number of neutrons = 40 – 18 = 22 neutrons
To determine the number of electrons, the charge of the atom must be considered. If a charge is not indicated, it can be assumed that the atom in question is neutral. A neutral atom has an equal number of positively charged protons and negatively charged electrons. Therefore, a neutral atom of argon has 18 electrons that balance the charge of its 18 protons, given by the atomic number.
Checklist
Here are reminders for how to determine the numbers of subatomic particles using information found in the periodic table:
Number of protons = atomic number
Number of neutrons = mass number – number of protons (atomic number)
Number of electrons = number of protons (atomic number) in a neutral atom
Isotopes
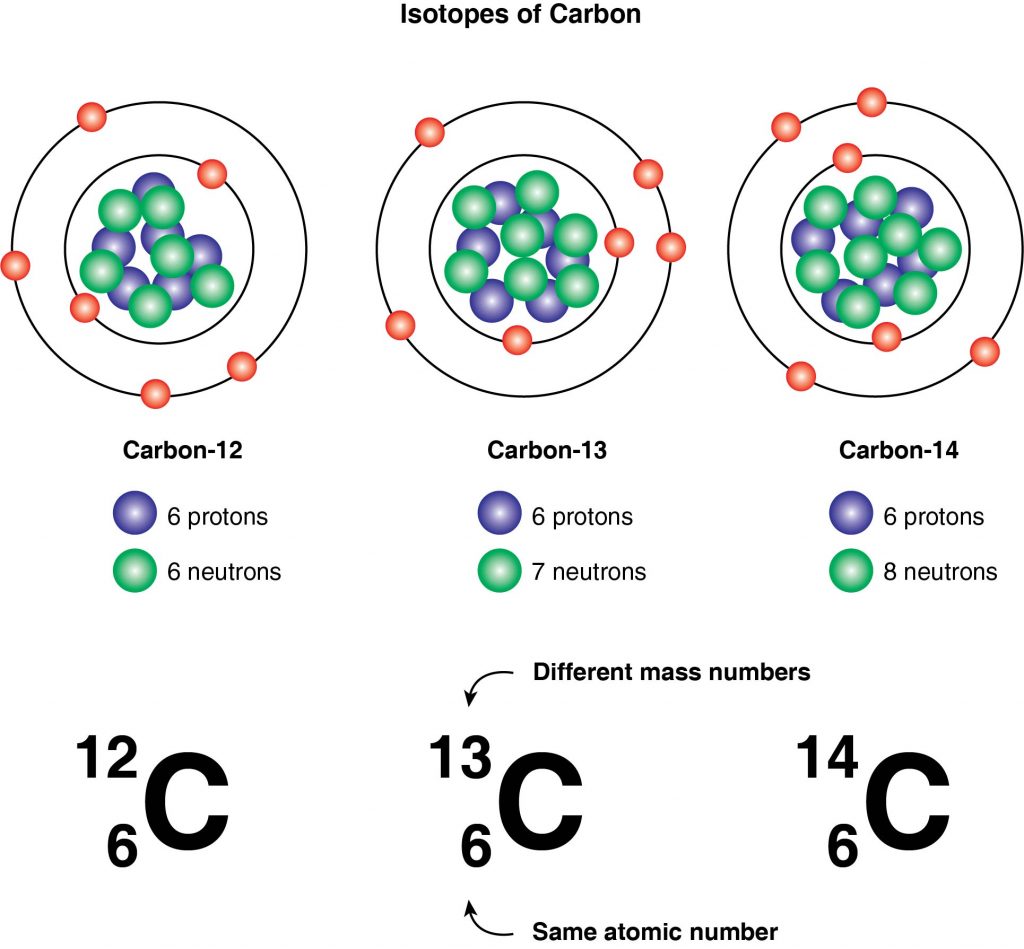
All atoms of an element have the same number of protons, but the number of neutrons may be different. Atoms that have the same number of protons but different numbers of neutrons are called isotopes. Because they have the same number of protons, they are the same element. However, because they contain different numbers of neutrons, their masses and mass numbers are different. The Bohr models for three isotopes of carbon are shown below.
All three isotopes have 6 protons because they are all different forms of carbon. They all have 6 electrons because these are neutral atoms of carbon, which means that the positive and negative charges balance each other. The different numbers of neutrons and the different masses differentiate these isotopes.
The isotopes can be named according to their masses. Carbon-12 has a mass number of 12, with 6 protons and 6 neutrons. Carbon-13 has a mass number of 13, with 6 protons and 7 neutrons. Carbon-14 has a mass number of 14, with 6 protons and 8 neutrons. The figure above shows how isotopes can be represented using the element symbols.
Isotopes are present in varying amounts. Carbon-12 makes up 98.93% of all carbon on Earth, and carbon-13 makes up 1.07%. Although carbon-14 exists, its amount is negligible. When calculating the average atomic mass, all isotopes are taken into account. In the periodic table, carbon has an atomic mass of 12.01 amu, which is extremely close to the mass of the most abundant isotope, carbon-12. Though not always true, the average atomic mass of an element is often closest to the mass of the most common isotope.
Let’s Review
- Scientific notation is used to make very large numbers and very small numbers easier to use.
- An atom is composed of protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, and electrons are found in the electron cloud that surrounds the nucleus.
- The number of protons in an atom determines its identity (which element it is).
- The mass number of an atom is determined by adding the number of protons and the number of neutrons.
- The charge of an atom is determined by the numbers of protons and electrons.
- Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses.
Subscribe to the online course to gain access to the full lesson content.
If your not ready for a subscription yet, be sure to check out our free practice tests and sample lesson at this link
