Acids and Bases
TEAS Science Lesson – Acids and Bases
In the previous TEAS science lesson you learned about chemical solutions. This TEAS science lesson introduces the properties of acids and bases, including the various theories that define them. It also covers acid-base reactions and the pH scale.
The following video will provide a review on Acids and Bases.
Nature of Acids and Bases
Acids are compounds that contain at least one hydrogen atom or proton (H+), which, when dissolved in water, can form a hydronium ion (H3O+). Acids dissolved in water generally have the following properties:
- Tastes sour
- Turn litmus red
- Act corrosive
Acids are found in a variety of substances, from vinegar to apple juice. The following table provides a list of common acids and their sources or applications.
| Name of Acid | Chemical Formula | Sources or Applications |
|---|---|---|
| Citric Acid | \(C_6H_8O_7\) | Citrus fruits such as oranges and lemons |
| Lactic Acid | \(C_3H_6O_3\) | Yogurt and buttermilk |
| Acetic Acid | \(C_2H_4O_2\) or \(CH_3COOH\) | Nail polish remover and vinegar |
| Hydrochloric Acid | \(HCl\) | Stomach |
| Phosphoric Acid | \(H_3PO_4\) | Detergents and soft drinks |
| Nitric Acid | \(HNO_3\) | Fertilizers |
Bases are compounds that form hydroxide ions (OH–) in a water solution. They also accept hydronium ions from acids. Bases dissolved in water generally have the following properties:
- Slippery in solution
- Very corrosive
- Turn litmus blue
- Taste bitter
Like acids, bases have many applications. The following table provides examples of common bases and how they are used.
| Name of Base | Chemical Formula | Applications |
|---|---|---|
| Sodium Hydroxide | \(NaOH\) | Soap, oven cleaners, and textiles |
| Potassium hydroxide | \(KOH\) | Soap and textiles |
| Ammonia | \(NH_3\) | Cleaning agents and fertilizers |
| Magnesium Hydroxide | \(Mg(OH)_2\) | Laxatives and antacids |
Acidic solutions have more hydrogen ions than hydroxide ions, whereas basic solutions have more hydroxide ions than hydrogen ions. All water solutions have both ion types, but the relative numbers dictate whether an aqueous solution is acidic, basic, or neutral. Anything that is dissolved in water is an aqueous solution. Neutral solutions are neither acidic nor basic, meaning that an equal number of hydrogen and hydroxide ions are present. Pure water is an example of a neutral solution.
Water is the primary solvent used to create an aqueous solution. Thus, it is important to understand how pure water behaves in solution. A small fraction of water molecules breaks down to form hydronium and hydroxide ions. When two water molecules interact, one water molecule gives up a positively charged hydrogen ion to form a hydroxide ion. A hydronium ion forms when a water molecule accepts a hydrogen ion. The following equation illustrates this reaction:
\(2H_2O → H_3O^+ + OH^-\)
Keep In Mind

Substances that form ions in aqueous solutions are called electrolytes. As electrolytes, acids and bases are conductors of electricity in solution. This is because they contain dissolved ions.
Try these TEAS exam science questions
Acid and Base Classification
Recall that an acid produces hydrogen ions, and a base produces hydroxide ions. These compounds are defined as Arrhenius acids and bases. The Arrhenius theory explains how acids and bases form ions when dissolved in water. Take, for example, the acid HCl, shown in the equation below. When forming an aqueous solution of HCl, this acid dissociates, or splits, into hydrogen ions and chloride ions in water.
\(HCl (g) → H^+ (aq) + Cl^- (aq)\)
An Arrhenius base dissociates into hydroxide ions (OH-) in an aqueous solution. This is the case for sodium hydroxide, NaOH, as shown in the following equation:
\(NaOH (s) → Na^+ (aq) + OH^- (aq)\)
One limitation of this theory is that it does not account for acids and bases that lack a hydrogen or hydroxide ion in their molecular structure. Another way to define acids and bases is by using the Brønsted-Lowry theory. A Brønsted-Lowry acid is a hydrogen ion donor that increases the concentration of hydronium ions in solution. A Brønsted-Lowry base is a hydrogen ion acceptor that increases hydroxide ion concentration in solution. The term proton is used interchangeably with the term hydrogen ion.
When a base accepts a hydrogen ion, it produces a conjugate acid. When an acid donates a hydrogen ion, it produces a conjugate base. In the following example, ammonia is the base, but its conjugate acid is ammonium ion. What is the conjugate base for the acid?
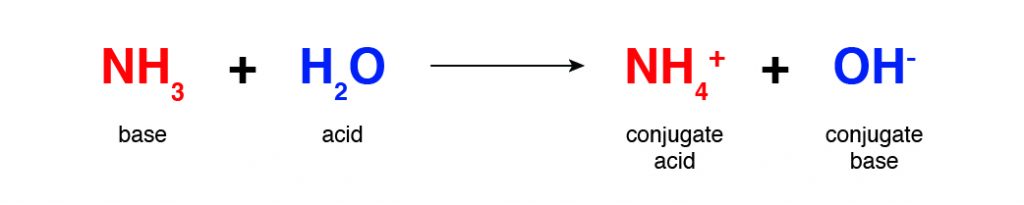
Be Careful
Free H+ ions do not float in aqueous solution. Rather, they bind with water to form \(H_3O^+\). However, it is not uncommon to see the two formulas, H+ and \(H_3O^+\), used interchangeably in chemical reactions.
The last theory about acids and bases is called the Lewis theory. This theory is based on electron movement during an acid-base reaction. A Lewis acid accepts a pair of electrons, while a Lewis base donates an electron pair.

Keep In Mind
When substances such as pure water act as an acid or a base, they are amphoteric.
Try this TEAS exam science question
Acid-Base Reactions
In an aqueous solution, a base increases the hydroxide concentration (OH–), while an acid increases the hydrogen ion (H+) concentration. Sometimes, neutralization reactions also occur. This type of reaction happens when an acid and a base react with each other to form water and salt. Salt is typically defined as an ionic compound that includes any cation except H+ and any anion except OH–. Consider the following example of a neutralization reaction between hydrobromic acid (HBr) and potassium hydroxide (KOH).
\(HBr + KOH → KBr + H_2O\)
In the above equation, one molecule of water forms in addition to the salt potassium bromide (KBr). There are instances where acid-base reactions must be balanced because more than one molecule of an acid or a base react to form products. This is the case for the reaction between hydrochloric acid and magnesium hydroxide, as shown below.
\(2HCl + Mg(OH)_2 → MgCl_2 + 2H_2O\)
When two molecules of hydrochloric acid react with magnesium hydroxide, two water molecules and one molecule of salt, MgCl2, form.

Be Careful
Not all neutralization reactions proceed in the manner where all reactants are in the aqueous phase. In some chemical reactions, one reactant may be a solid. The neutralization reaction can still proceed to completion.
Try this TEAS exam science question
Acid Base Strength and pH
Acids and bases can be classified according to their strength. This strength refers to how readily an acid donates a hydrogen ion. The strength of a base is determined by how readily it removes a hydrogen ion from a molecule, or deprotonates. Strong acids are also known as strong electrolytes, which means that they completely ionize in solution. Weak acids are weak electrolytes because they partially ionize in solution. The following diagram shows what happens to a strong or weak acid in an aqueous solution.

The maximum number of ions is produced when strong acids ionize. As shown in the following equations, the weak acid reaction is reversible (and incomplete) in aqueous solutions. This explains why weak acids produce fewer ions than strong acids.
Strong acid in solution: \( HNO_3 → H^+ + NO_3^- \)
Weak base in solution: \( NH_3 + H_2O ↔ NH_4^+ + OH^-\)
Like strong acids, strong bases fully dissociate in solution. They produce metal ions and hydroxide ions. Like weak acids, weak bases partially dissociate and participate in reversible reactions. The following table provides a list of common strong acids and bases and common weak acids and bases.
| Strong Acid | Weak Acid | Strong Base | Weak Base |
|---|---|---|---|
| Hydrochloric Acid \((HCl)\) | Hydrofluoric Acid \((HF)\) | Sodium Hydroxide \((NaOH)\) | Ammonia \((NH_3)\) |
| Nitric Acid \((HNO_3)\) | Carbonic Acid \((H_2CO_3)\) | Potassium Hydroxide \((KOH)\) | Methylamine \((CH_3NH_2)\) |
| Perchloric acid \((HClO_4)\) | Phosphoric Acid \((H_3PO_4)\) | Calcium Hydroxide \((Ca(OH)_2)\) | Hydrazine \((N_2H_4)\) |
| Sulfuric Acid \((H_2SO_4)\) | Acetic Acid \((C_2H_4O_2\) or \((CH_3COOH)\) | Lithium Hydroxide (LiOH) | Pyridine \((C_5H_5N)\) |

Be Careful
Ammonia is a weak base even though it does not have a hydroxide ion \((OH^-)\) in its chemical formula. It will accept a proton and form hydroxide ions in aqueous solutions.
Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.

Try this TEAS exam science question
Let’s Review
- Acids and bases exhibit unique properties when dissolved in water.
- Acids donate protons (in the form of hydrogen ions) and bases accept protons (or hydrogen ions) in solution.
- Acids generally taste sour, turn litmus paper red, and act corrosive.
- Bases generally taste bitter, turn litmus paper blue, are very corrosive, and will be slippery in solution.
- A neutralization reaction occurs when an acid and a base react to form a salt and water.
- Strong acids completely ionize in solution, and strong bases fully dissociate in solution.
- Weak acids and weak bases only partially dissociate in solution.
- The pH of a solution determines how acidic or basic it is. A pH lower than 7 means a solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
Subscribe to the online course to gain access to the full lesson content.
If your not ready for a subscription yet, be sure to check out our free practice tests and sample lesson at this link
