An Introduction to Chemistry
TEAS Test Science Prep – An Introduction to Chemistry
In this TEAS test science prep lesson we discuss matter which is anything that takes up space and has mass. Chemistry is the study of matter, its properties, and properties of the substances that matter creates. At this point, we understand that cells are the basic subunits of all living things. But instead of analyzing cells on a grander scheme as in anatomy and physiology, we can study cells on a smaller, molecular level. This section will provide further information on atoms and their binding properties, which create the molecules and compounds that further comprise the cell and thus the organism.
An Overview of Matter
- Matter is anything that takes up space and has mass.
- An atom is the basic unit of matter. Atoms are also known as elements.
- An atom is composed of protons, neutrons, and electrons. Protons and neutrons are found in the nucleus, and electrons are found in the electron cloud that surrounds the nucleus.

- The number of protons in an atom determines its identity (which element it is).
- The mass number of an atom is determined by adding the number of protons and the number of neutrons.
- The charge of an atom is determined by the numbers of protons and electrons.
- Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses.
📝 Click here for the corresponding notes on Atoms, Elements, & the Periodic Table
The Periodic Table of Elements
All known atoms/elements are organized into the Periodic Table of Elements. Specific trends exist within the Periodic Table.

- Each element has a name and chemical symbol, usually a letter abbreviation, unique to the atom.
- The periodic table provides an atom’s atomic number and atomic mass. Elements in the table are listed in order of increasing atomic mass.
- Periodic tables differ in the information they provide, and an example of a block is shown. This block shows the name of the element and its chemical symbol, which is an abbreviation for the name. The chemical symbol is one, two, or three letters with the first letter capitalized and all subsequent letters letter lowercase. The symbol for the element argon is Ar.
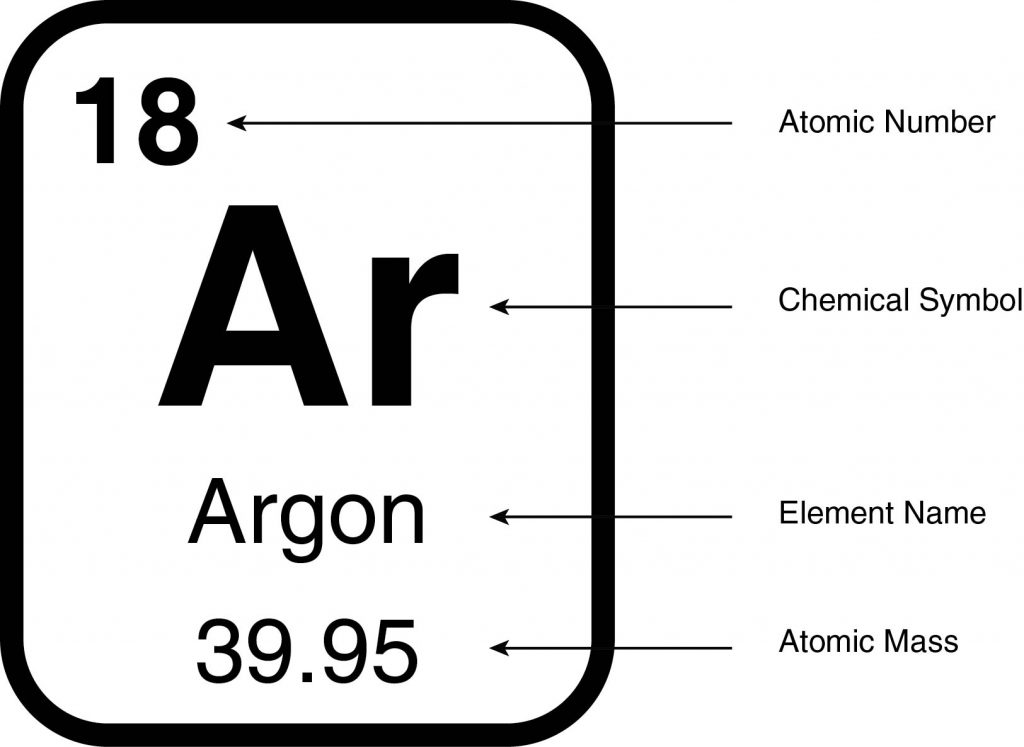
- Each element is assigned an atomic number. The atomic number is equal to the number of protons in a single atom of that element and is how an element is identified. Argon, for example, has an atomic number of 18. Therefore, every atom of argon has 18 protons, regardless of how many neutrons or electrons it has.
- Electrons are located in an atom’s electron cloud, divided into shells. Atoms are constantly seeking to gain, share, or give up electron in order to have a full shell.
- Valence electrons are the unpaired electrons in the outermost shell.
- Atoms in the same column, or group, have the same number of valence electrons in their outermost shell. An atom’s outermost shell is equal to the row that the element is located in.
- Exceptions to the trends exist. Transition metals (atoms in columns 3-12) have 2 valence electrons.
- Atoms in columns 13-18 have the number of valence electrons equal to the column number minus 10. Noble gasses have 8 valence electrons and a full outermost shell.
- Helium is considered a noble gas because it has a full outermost shell. However, it only has 2 valence electrons in the first electron shell level.
Did You Know?

While many elements have chemical symbols that resemble their names, like argon (Ar), some elements have chemical symbols that are different from their names. This is because the symbols are derived from either the Latin or the Greek names for the elements rather than the English names. The symbol for sodium is Na because the Latin name for the element is natrium.
States of Matter
- Solids, liquids, gases, and plasmas differ from one another in the amount of energy that the particles have and the strength of the cohesive forces that hold the particles together.
- Cohesion is the tendency of particles of the same kind to stick to each other.
- A solid has the lowest amount of energy because its particles are packed close together. Liquids have more energy than a solid, and gases have more energy than solids or liquids because the cohesive forces are very weak.
- A substance can undergo a phase change if it either absorbs or releases enough energy.

- Heating and cooling curves show the temperature of a substance as heat is consistently added or removed.
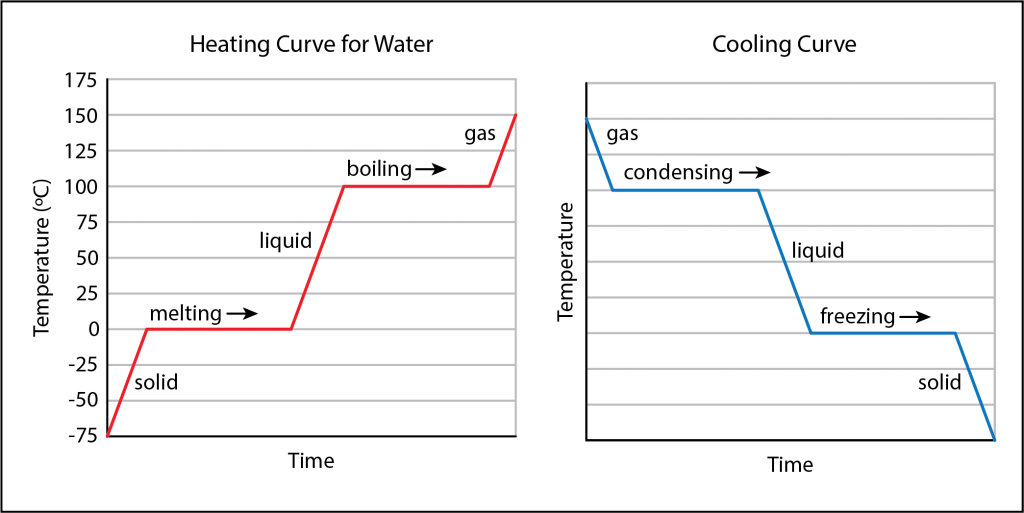
- The only time a substance experiences an increase or decrease in temperature is when it is entirely in one state of matter.
- As a substance changes states, its temperature remains constant. Any energy that is absorbed or released is used to change the way in which the particles interact with one another.
Try this TEAS test practice science question
Properties of Matter
- The difference between physical and chemical properties is that chemical properties involve a change in a substance’s chemical composition and physical properties do not.
- Physical properties can be categorized as either extensive or intensive properties. Intensive properties do not depend on the amount of matter present, such as color or density. Extensive properties do depend on the amount of a sample present, such as mass and volume.
- Water is considered a polar molecule, a molecule where one end is slightly negative and the other end is slightly positive.
- Cohesiveness is the attraction of water to itself, and adhesiveness is the attraction of water to other substances. Adhesion is the property that allows for water to travel against the force of gravity within a plant.
- Diffusion is the tendency of molecules and ions to move toward areas of lower concentrations until a substance is uniform.
- Osmosis is the diffusion of water and the movement of molecules from an area of high concentration to an area of lower concentration.
Try this TEAS test practice science question
Chemical Bonds
- The periodic table of elements organizes elements in order of increasing atomic mass and by other characteristics. Elements have the number of energy levels equivalent to the row they are in. Elements in the same column of the periodic table have the same number of valence electrons in the last energy level.
- As stated in the octet rule, any atom that does not have a stable electron configuration of eight valence electrons will lose, gain, or share electrons to become stable.
- Exceptions to the octet rule include hydrogen and helium, which are stable when they have two valence electrons. This is because the first energy level of the electron cloud can fit two electrons.
- Ionic bonds are formed when electrons are transferred from a metal atom to a nonmetal atom. When an atom gains electrons, it becomes an anion, because it has a net negative charge since it gains negatively charged particles. When an atom loses electrons, it’s called a cation because it has a net positive charge since the newly formed ion will have more protons than electrons.
- Covalent bonds are formed when two atoms share electrons. When two atoms need to share more than one pair of electrons, multiple bonds form. If two pairs are shared, a double bond forms. If three pairs are shared, a triple bond forms.


- The difference in the electronegativities of the two atoms determines if electrons are shared equally, forming a nonpolar covalent bond, or shared unequally, forming a polar covalent bond.
- Lewis structures are diagrams of atoms to represent bonding. Valence electrons and lone-pair electrons, or electrons not involved in bonding, are represented with dots while bonding patterns are represented with lines.
Try this TEAS test practice science question
Chemical Solutions
- A mixture is when elements and compounds are physically, but not chemically, combined.
- A homogeneous mixture is when substances mix evenly and it is impossible to see individual components. A heterogeneous mixture is when the substances mix unevenly and it is possible to see individual components.
- A solution is a type of homogeneous mixture that is formed when a solute dissolves in a solvent.
- The concentration of a solution is the amount of a substance in a given amount of solution. An unsaturated solution has the ability to dissolve more solute and a saturated solution has already reached the limit of solute it can dissolve.
- The law of conservation of mass states that matter cannot be created or destroyed, but it can change forms.
- Chemical reactions occur when reactants combine, break apart, or rearrange to form products.
- Chemical equations represent chemical reactions using formulas and symbols.
- Chemical equations must be balanced to obey the law of conservation of mass to ensure that all atoms at the beginning of the reaction in the reactants are accounted for at the end in the products.
- Chemical reactions can be classified as synthesis, decomposition, single-replacement, double-replacement, or combustion based on the reactants and products.

- Energy diagrams show how the energy of the species involved in the reaction changes as the reaction progresses.

Try this TEAS test practice science question
Acids and Bases
- Acids and bases exhibit unique properties when dissolved in water.
- Acids donate protons (in the form of hydrogen ions) and bases accept protons (or hydrogen ions) in solution.
- Acids generally taste sour, turn litmus paper red, and act corrosive.
- Bases generally taste bitter, turn litmus paper blue, are very corrosive, and will be slippery in solution.
- A neutralization reaction occurs when an acid and a base react to form a salt and water.
- Strong acids completely ionize in solution, and strong bases fully dissociate in solution.
- Weak acids and weak bases only partially dissociate in solution.
- The pH of a solution determines how acidic or basic it is. A pH lower than 7 means a solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
In the next TEAS test science prep lesson you will learn about states of matter.

Try this TEAS test practice science question
Subscribe to the online course to gain access to the full lesson content.
If your not ready for a subscription yet, be sure to check out our free practice tests and sample lesson at this link
